Research
Research
Given the axial position of plants as a source of food for human beings and animals, a growing movement has emerged during the past two decades to address the role of agricultural practices in solving social problems in areas with low production. With the continuously growing population on earth, improving plant yield is one among other means that may positively contribute to sustain our lives and societies. To this end, in the mid- and long-term, shall our discoveries go outside the limits of basic science to propose new concepts and ways to raise crop yield, by influencing applied biological and environmental fields.
Leaves are central organs for photosynthesis (biomass production), and sensing and responding to environmental signals (photoperiod, temperature…). Therefore, understanding leaf development, in its wider meaning, is central to understand the way of plant life. One of our goals is to understand how leaf-size is regulated, as this is poorly understood. In leaves, the coordination of cell proliferation and expansion, fundamental to proper organogenesis, was suggested by a "COMPENSATION" phenomenon, whereby a decrease in cell proliferation triggers enhanced cell expansion. To elucidate the mechanisms of compensation at the molecular level, we are using many mutant lines that exhibit compensation phenotype. By combining genetic, molecular biology and biochemical tools, we are now dissecting not only the role of each single gene, but the network behing the leaf growth and development dynamism.
Achievements & Current Research Topics
Three different modes in "COMPENSATION"
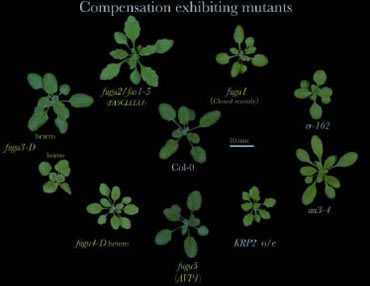
We have analyzed collectively 8 different mutant and transgenic plants that exhibit compensation (Ferjani et al., 2007). Through kinematic analyses of cell size in leaves, we identified three different modes of post-mitotic expansion.
Class I, enhanced post-mitotic cell expansion rate. Class II, extended post-mitotic cell expansion period. Class III, increased size of dividing cells.
* Cloning of the gene mutated in fugu1 is now ongoing.
Figure: slightly modified with permission from Ferjani et al. (2007); www.plantphysiol.org, "Copyright American Society of Plant Biologists".
The class III compensation, of the KRP2 overproducing lines, depends on V-ATPase activity in proliferating cells

In this study, we unambiguously demonstrated that increased V-ATPase activity is a positive regulator of Compensated Cell Enlargement (CCE) in KRP2 o/e lines, but not in other compensation-exhibiting mutants (Ferjani et al., 2013).
This study provides new insights into one major pathway that specifically affects CCE. To our knowledge this report is the first to provide a fundamental link between reduced cell cycling and enhanced cell expansion during leaf development.
* Does this increase in V-ATPase activity alone explains all the similarities shared by transgenics overexpressing different plant KRPs?.
Figure: slightly modified with permission from Ferjani et al. (2007); www.plantphysiol.org, "Copyright American Society of Plant Biologists".
The ATM-dependent DNA damage response acts as an upstream trigger for compensation in the fas1/fugu2 mutant

In this study, the ATM-dependent DNA damage response has been identified as an upstream trigger in fas1/fugu2, which delays the cell cycle progression and promotes the entry into the endocycle, resulting in CCE (Hisanaga T, Ferjani A et al., 2013).
Figure: slightly modified with permission from Ferjani et al. (2007); www.plantphysiol.org, "Copyright American Society of Plant Biologists".
The first elucidation of the biological roles of the H+-pyrophosphatase

In this study, we show that the Arabidopsis thaliana fugu5 mutant, a loss-of-function mutant of the vacuolar H+-pyrophosphatase (H+-PPase), failed to support heterotrophic growth after germination. Importantly, we found that exogenous supplementation of sucrose or the specific hydrolysis of the cytosolic pyrophosphate (PPi) by the the cytosolic-type inorganic pyrophosphatase1 (IPP1) enzyme from budding yeast rescued fugu5 phenotypes. Furthermore, we revealed that the peroxisomal β-oxidation activity, dry seed contents of TAGs, and their breakdown were unaffected in fugu5. By contrast, fugu5 mutants contained ~2.5-fold higher PPi and ~50% less sucrose than the wild type.
All together, these findings provide solid evidence that gluconeogenesis is inhibited due to the high levels of PPi in the cytosol. This study further suggested that the hydrolysis of cytosolic PPi, rather than vacuolar acidification, is the major function of H+-PPase in vivo at the early stages of postgerminative development (Ferjani et al., 2011; 2012). This paper was introduced as an In Brief Article "A surprising role for vacuolar pyrophosphatase" by The Plant Cell (Bertoni, 2011).
Figure: Arabidopsis fugu5 mutant is defective in the vacuolar H+-PPase AVP1. In the absence of an exogenous nutrient source (sucrose), fugu5 mutant cotyledons display altered morphology (top panels) plus changes in cell size and number. Expression of the yeast cytosolic PPase IPP1 rescued both the morphological phenotype (bottom panels) and cellular abnormalities. Bar = 2 mm. Figure: modified with permission from Ferjani et al. (2011); www.plantcell.org, "Copyright American Society of Plant Biologists".
